




MBeagle dog, Mini-pig and Cynomolgus monkey.
Oral (PO), intravenous (IV), intraarterial (IA), intramuscular (IM), intraperitoneal (IP), intratracheal (IT), subcutaneous (SC), transdermal (TD), intranasal (IN), IV infusion, SC infusion, rectal, and topical administration.
Bile-duct cannulation (BDC), jugular cannulation (JVC), portal vein cannulation (PVC), carotid artery cannulation (CAC), mesenteric lymph duct cannulation, bile-duct ligation, and castration.
Whole blood, plasma, serum, bile, feces, urine, cerebrospinal fluid, bone marrow, lymph node, other fluids and tissues.
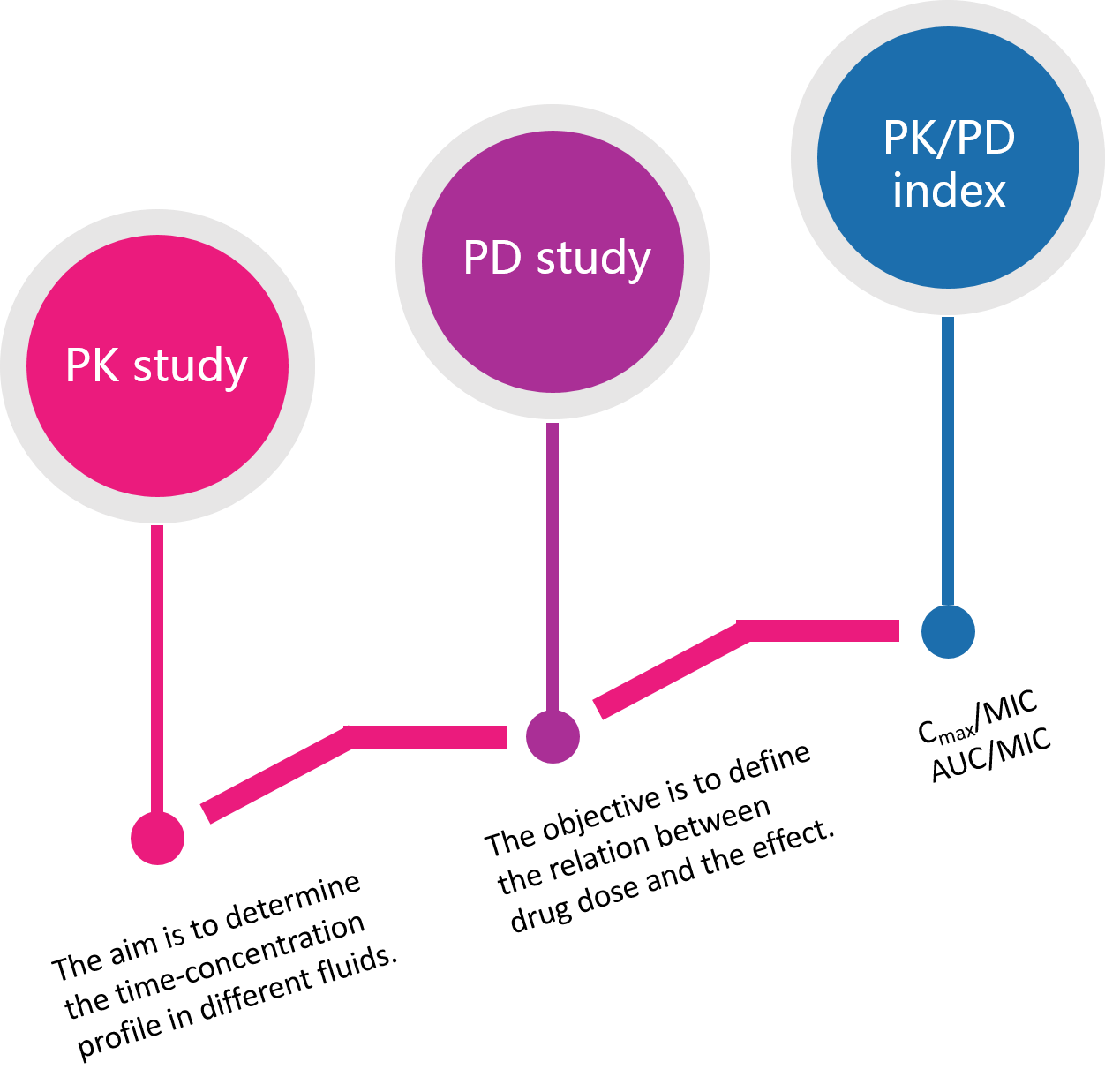
PK/PD analysis is a key aspect of drug development. Understanding the bioavailability, exposure, half-life, clearance, and metabolism of a drug may determine the success or failure in the clinic. Creative Bioarray offers in vivo PK/PD services by utilizing our extensive expertise and models to support drug discovery and preclinical development for small molecules, peptides, nucleosides/nucleotides and biologics. All in-life work of PK/PD studies are carried out in AAALAC-approved animal facilities and follow IACUC-approved protocols. Whatever your needs, we work with you to identify the most appropriate models and to provide the customized study designs.
Contact Us
Inquiries